Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
Difference between revisions of "Regulatory Experimentation Expense Fund"
Roaa.hamed (talk | contribs) |
Roaa.hamed (talk | contribs) m |
||
| Line 1: | Line 1: | ||
| − | |||
[[FR: Fonds de dépenses d’expérimentation réglementaire]] | [[FR: Fonds de dépenses d’expérimentation réglementaire]] | ||
<div style="background:#1F402B; | <div style="background:#1F402B; | ||
| Line 116: | Line 115: | ||
|Health Canada is developing a sandbox to test new regulatory approach for MLMDs. Currently, MLMD can be marketed in Canada if the device algorithm behaviour is locked to remain the same overtime as what was presented in the product’s market authorization application. Any change to the algorithm behaviour requires the submission of an amendment to the licence. Health Canada wants to test a new regulatory approach that would use a different method of risk management than requiring a licence amendment so that the device can change its behaviour over time as it learns from new data it acquires over time. The current project scope is limited to accessing the legislative authorities to establish the sandbox and developing the sandbox application scheme. | |Health Canada is developing a sandbox to test new regulatory approach for MLMDs. Currently, MLMD can be marketed in Canada if the device algorithm behaviour is locked to remain the same overtime as what was presented in the product’s market authorization application. Any change to the algorithm behaviour requires the submission of an amendment to the licence. Health Canada wants to test a new regulatory approach that would use a different method of risk management than requiring a licence amendment so that the device can change its behaviour over time as it learns from new data it acquires over time. The current project scope is limited to accessing the legislative authorities to establish the sandbox and developing the sandbox application scheme. | ||
|} | |} | ||
| + | __NOTOC__ | ||
| + | __NOEDITSECTION__ | ||
Revision as of 09:58, 2 November 2023
Centre for Regulatory Innovation: Regulatory Experimentation Expense Fund
| Home | Events | What is Regulatory Experimentation? | Regulatory Experimentation Expense Fund | Regulators' Capacity Fund | Completed CRI Supported Projects | Tools and Resources |
|---|
Project Eligibility
The REEF is a fund to help regulators innovate or support innovation in the marketplace by experimenting with:
- Innovative approaches to any stage(s) of the regulatory lifecycle (issue definition and instrument choice, regulatory development, administration, compliance/enforcement, and review/evaluation)
- Market innovations (e.g., products, business models, services.)
All stages of regulatory experimentation are supported, including:
- determining whether an experiment would be the right approach (NEW)
- developing a regulatory experiment (e.g., scope, evidence needs, experimental process, data collection methods) (NEW)
- conducting a regulatory experiment
What can the REEF offer?
Regulators can receive:
- financial support to offset expenses associated with experimenting. Up to $1,400,000 per fiscal year with multi-year funding is available.
- technical advice and guidance to help you navigate your specific situation.
Interested in applying? Want to learn more?
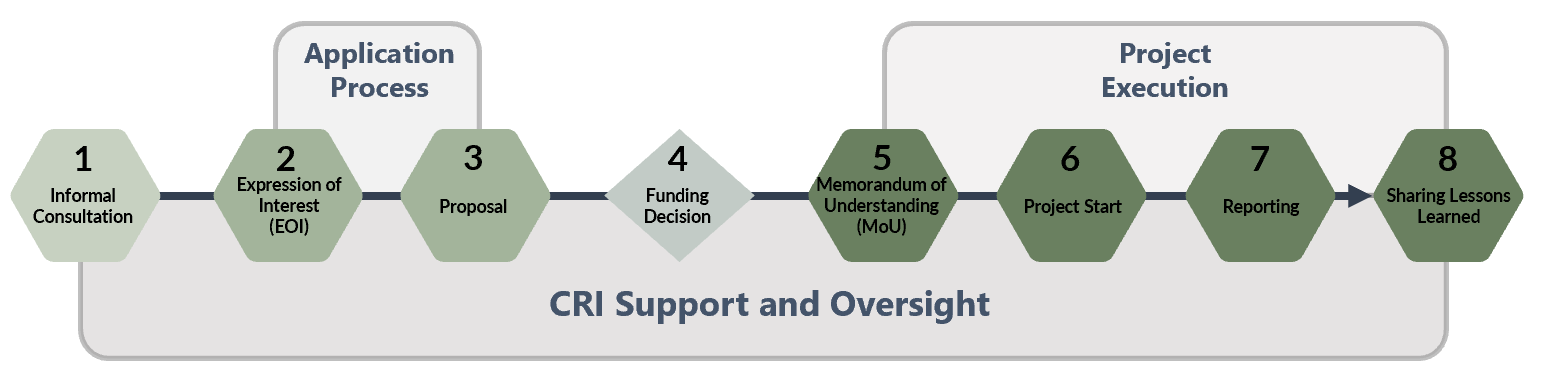
The first step is to contact the Centre for Regulatory Innovation to request an informal consultation to determine whether and how the REEF can best support your goals.
Several different types of projects have been eligible for the REEF – read more here.
The REEF Process
Step 2: Expression of Interest Submission
The departments submit a high-level Expression of Interest (EOI) that is used by the CRI to determine the eligibility of the project. To be eligible for funding, proposed experiments must clearly demonstrate how their funding request meets the criteria as outlined in the REEF Guide. It is highly recommended that applicants informally confirm the eligibility of their project by scheduling an informal consultation with the CRI before completing an EOI. EOI submissions are due January 16, 2024.
Any Government of Canada regulator may apply for funding. Priority will be given to experiments where regulators have identified collaboration with regulated entities or other businesses with the aim of bringing applications of new and emerging technologies into the Canadian marketplace or enhancing competitiveness.
The EOI provides a brief overview of the proposed experiment or pre-experimentation project and amount of funding requested. The EOI should not take more than a few hours to complete. If you have questions on how to complete the EOI, it is recommended that you reach out to the CRI. EOIs should be submitted by email to cri-cir@tbs-sct.gc.ca. The CRI will contact applicants as required if clarifications on the proposed experiment or pre-experimentation project are needed. Applicants with an eligible EOI will be invited to submit a proposal.
Step 3: Proposal Submission
Successful EOI applicants will be invited to complete the second stage of the application process which is the submission of a full proposal. Before developing a proposal, applicants should obtain the support of their Director General or above. The CRI supports applicants throughout the proposal development process. Regulators should refer to the Regulators’ Experimentation Toolkit to assist in identifying, designing, and carrying out regulatory experiments and sandboxes.
Proposals will be assessed, and final funding decisions will be determined by an interdepartmental Steering Committee, made up of representatives from TBS, Privy Council Office, Innovation Science and Economic Development, Transport Canada, Environment and Climate Change Canada, Health Canada, and the Community of Federal Regulators.
Step 4: Funding Decision
Funding will be available from April 2023 upon completion of a MOU with the successful applicant and TBS, and is made available on a cost recovery basis.
The department is responsible to forecast their expenses and include the spending schedule in the MOU. The department will be expected to submit scheduled invoices to TBS to recover the expenses incurred as well as submitting financial forecast to allow TBS to decommit the funds that the department no longer plans on recovering.
Application Documents
- REEF Guide
- Regulators’ Experimentation Toolkit
- Expression of Interest (EOI) Template
- Proposal Template
REEF Ongoing Projects
| Click here to see a list of the CRI’s completed projects and experimentation reports | ||
|---|---|---|
| Department/ Agency | Project Title | Project Description |
| Transport Canada (TC) | Light Sport Aircraft | This experiment as proposed by TC involves setting up a sandbox to determine if Light Sport Aircraft, with appropriate conditions, are as reliable as other aircraft currently being used for the purpose of flight training and if the quality of training is as good, or better, than other aircraft currently used for pilot training. The experiment will also inform Transport Canada on potential additional conditions/measures that should be included in future exemptions or regulatory amendments. |
| Innovation Science and Economic Development (ISED) – Standards Council of Canada (SCC) | Piloting an Accreditation Program for the Assessment of Artificial Intelligence Management Systems (AIMS) | The purpose of this experiment is to pilot a prototype accreditation program to understand whether the main SCC Requirements and Guidance document that works in conjunction with ISO/IEC 17021-1:2015 Conformity assessment is clear and appropriate and obtain feedback from the certification bodies and Artificial Intelligence (AI) companies about the program. This information is necessary to improve and refine the prototype.
The SCC is also looking to gather information on whether the AIMS could have an impact on the level of risk of AI products to inform potential use of AIMS for regulators. |
| Transport Canada (TC) | Aviation E-Licensing Pilot | This is a follow up to previous experiment to test a new QR code prototype with pilots of an air operator within Canada for effectiveness. The sandbox experiment will test if the digital solution lowers the cost of compliance and improve compliance with respect to air operators’ obligation to track employee compliance with licensing requirements. |
| Health Canada (HC) | Machine Learning Enabled Medical Device (MLMD) Sandbox Development | Health Canada is developing a sandbox to test new regulatory approach for MLMDs. Currently, MLMD can be marketed in Canada if the device algorithm behaviour is locked to remain the same overtime as what was presented in the product’s market authorization application. Any change to the algorithm behaviour requires the submission of an amendment to the licence. Health Canada wants to test a new regulatory approach that would use a different method of risk management than requiring a licence amendment so that the device can change its behaviour over time as it learns from new data it acquires over time. The current project scope is limited to accessing the legislative authorities to establish the sandbox and developing the sandbox application scheme. |