Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
Difference between revisions of "Regulatory Experimentation Expense Fund draft"
| Line 127: | Line 127: | ||
| | | | ||
===<small>Expression of Interest Assessment</small>=== | ===<small>Expression of Interest Assessment</small>=== | ||
| − | <div style="background-color: #E3F2FD; padding: 5px; border-radius: 5px; margin: 15px 0;"> | + | * |
| + | |<div style="background-color: #E3F2FD; padding: 5px; border-radius: 5px; margin: 15px 0;"> | ||
After the submission deadline, there will be a review period where: The CRI team will provide feedback on submitted EOIs, applicants may be asked to update or clarify aspects of their EOI. This iterative process ensures all EOIs are as strong as possible before final assessment | After the submission deadline, there will be a review period where: The CRI team will provide feedback on submitted EOIs, applicants may be asked to update or clarify aspects of their EOI. This iterative process ensures all EOIs are as strong as possible before final assessment | ||
</div> | </div> | ||
Revision as of 10:14, 6 September 2024
| Home | Regulatory Sandboxes | What is Regulatory Experimentation? | Regulatory Experimentation Expense Fund | Regulators' Capacity Fund | CRI Supported Projects | Tools and Resources |
|---|
|
|
Project EligibilityThe Regulatory Experimentation Expense Fund (REEF) supports federal regulators in innovating or facilitating innovation in the marketplace by experimenting with:
All stages of regulatory experimentation are supported, including:
New this year: Expressions of interest are now accepted for Impact Canada challenge prize, for challenges intended to inform regulatory development. The REEF is open to all Government of Canada regulators. |
What can the REEF offer?
Regulators can receive:
Financial Support
- Up to $1,400,000 per fiscal year, with multi-year funding available, to cover expenditures considered necessary to support the purpose of the proposed regulatory experiment or pre-experiment
- Funds transferred as operations and maintenance funds (O&M) on a cost recovery basis
- The Treasury Board of Canada Secretariat reserves the right to make the final determination on eligible expenditures
Important Update: Due to multiple ongoing multi-year projects, funding for the new round of REEF will be approximately $400K. This limited budget will be allocated based on the most compelling proposals. Early consultation is recommended to align with current funding availability.
Note: Funds cannot be used to award a financial prize for an Impact Canada challenge prize. Prize awards require grants and contributions from the applicant.
Technical Support
- Expert advice and guidance to help navigate your specific regulatory context
Eligibility Criteria
To be eligible for the REEF, proposed regulatory projects must meet the following criteria:
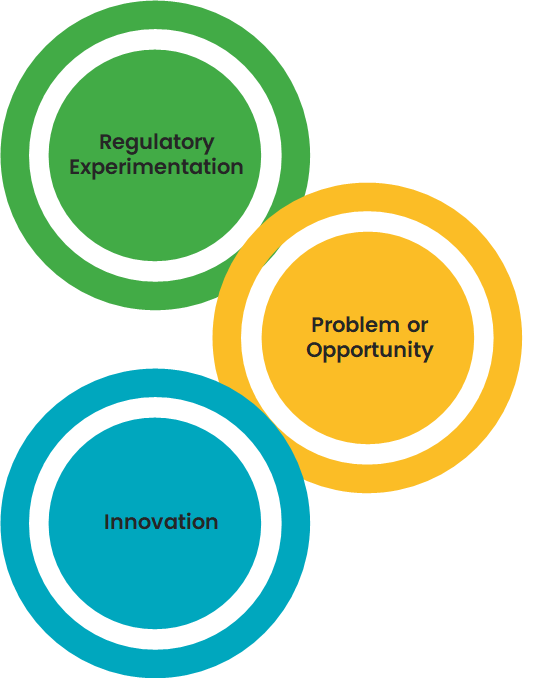
Regulatory Experimentation: The project must be about regulatory experimentation, either:
a. Conducting a regulatory experiment, or
b. Supporting pre-experimentation activities (developing an experiment or determining if experimentation is appropriate)
Problem or Opportunity: The project must address at least one of the following:
- A defined industry need
- A technological challenge
- A market opportunity
Innovation: The project must support innovation in regulatory approaches or in the marketplace.
Note: For an Impact Canada challenge prize:
- The proposed project does not need to be an experiment
- Eligibility criterion: Applicants must outline how their challenge will inform regulatory development.
Webinar: Regulatory Innovation Tools (December 13, 2023)
For further insight into the eligibility criteria and examples of successful projects, please watch the webinar on Regulatory Innovation Tools from December 13, 2023. This webinar provides valuable information on how to meet the criteria and enhance your proposal.
Click to access presentation materials:Regulatory Innovation Tools Webinar Deck
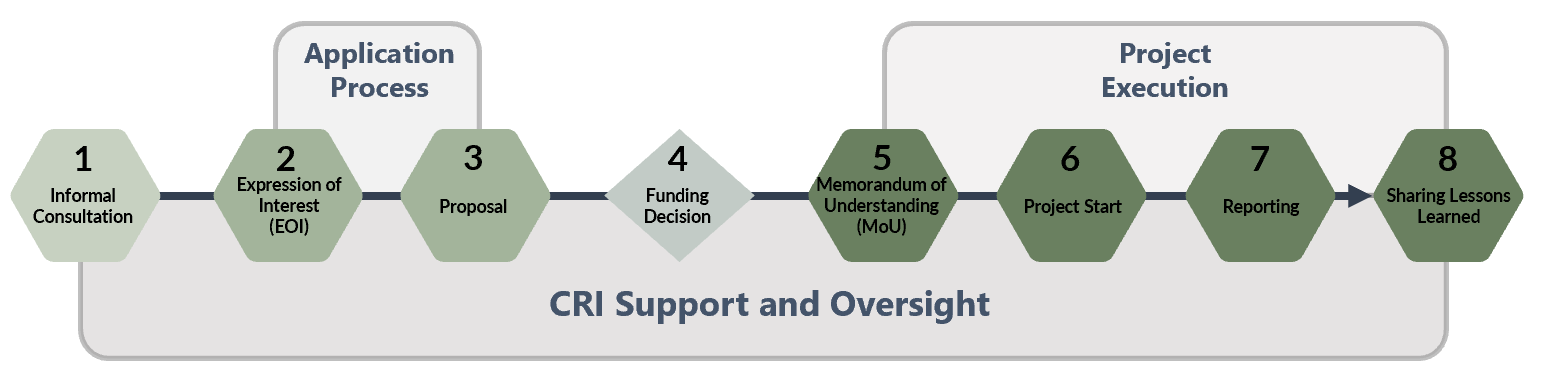
The Process
| Step | Description | |
|---|---|---|
| 1. |
Informal Consultation |
Before submitting an EOI, consult with the CRI to confirm project eligibility. Email: cri-cir@tbs-sct.gc.ca Note: For challenge prize projects, please mention this in your email. |
| 2. |
Expression of Interest |
|
| 3. |
Expression of Interest Assessment |
After the submission deadline, there will be a review period where: The CRI team will provide feedback on submitted EOIs, applicants may be asked to update or clarify aspects of their EOI. This iterative process ensures all EOIs are as strong as possible before final assessment When there are many high-quality Expressions of Interest (EOIs), the CRI will consult the SC to prioritize those that:
|
| 4. |
Proposal Submission |
|
| 5. |
Proposal Assessment |
For proposal assessment, the Steering Committee (SC) will:
|
| 6. |
Funding Decision |
|
| 7. |
Memorandum of Understanding |
|
| 8. |
Lessons Learned and Knowledge Sharing |
|
Application Documents
| Document | Description |
|---|---|
| Regulators' Experimentation Toolkit | Toolkit for regulatory experiments and sandboxes |
| Expression of Interest (EOI) Template | Template for submitting your initial interest |
| Proposal Template | Template for full project proposal |
Ongoing Projects
| Department/Agency | Project Title | Project Description |
|---|---|---|
| Transport Canada (TC) | Light Sport Aircraft |
Transport Canada is conducting a regulatory experiment to assess the suitability of Light Sport Aircraft (LSA) for use in flight training. The project aims to evaluate LSA certified by the European Union Aviation Safety Agency in Canadian flight training environments. Working with selected flight schools, Transport Canada will gather data on aircraft performance, reliability, and training quality. The experiment seeks to inform potential updates to regulations, potentially allowing more modern and environmentally friendly aircraft options for pilot training while maintaining safety standards. |
| Health Canada (HC) | Advanced Therapeutic Products Framework for CAR-T Products |
Health Canada is developing a regulatory sandbox for Chimeric Antigen Receptor T-cell (CAR-T) therapy products manufactured at the point of care. This experiment aims to test a co-creation process for developing tailored regulatory requirements under Health Canada's Advanced Therapeutic Products pathway. The project will involve collaboration with various stakeholders to design market access authorization requirements for these innovative cancer treatments. The goal is to create an adaptive regulatory framework that maintains safety standards while facilitating faster access to new therapies. |
| Canada Energy Regulator (CER) | Assessing Compliance of Potential New Requirements to Prevent and Address Impacts to Indigenous Rights and Interests: A Regulatory Experiment |
The Canada Energy Regulator (CER) is conducting a regulatory experiment to develop and test new requirements for preventing and addressing impacts to Indigenous rights and interests related to energy infrastructure projects. The experiment aims to enhance Indigenous involvement in compliance verification and oversight processes. Using a co-creation approach with Indigenous communities and other stakeholders, the CER will develop tailored regulatory requirements and test a joint compliance verification process. The project seeks to strengthen measures to protect Indigenous rights while informing updates to CER's regulatory framework. |
| View list of completed projects and experimentation reports | ||
Questions?
If you have any questions, please contact the CRI at: cri-cir@tbs-sct.gc.ca.