Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
Difference between revisions of "Regulatory Experimentation Expense Fund"
m |
|||
| (24 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File: | + | [[File:REEF Page.png|alt=|frameless|1062x1062px]]{{DISPLAYTITLE:<span style="position: absolute; clip: rect(1px 1px 1px 1px); clip: rect(1px, 1px, 1px, 1px);">{{FULLPAGENAME}}</span>}} |
| − | {{DISPLAYTITLE:<span style="position: absolute; clip: rect(1px 1px 1px 1px); clip: rect(1px, 1px, 1px, 1px);">{{FULLPAGENAME}}</span>}} | ||
[[FR: Fonds de dépenses d’expérimentation réglementaire]] | [[FR: Fonds de dépenses d’expérimentation réglementaire]] | ||
<div style="background:#6E8B7B; | <div style="background:#6E8B7B; | ||
| Line 6: | Line 5: | ||
font-family:Arial; | font-family:Arial; | ||
padding: 5px 5px 10px 25px;"> | padding: 5px 5px 10px 25px;"> | ||
| − | |||
</div> | </div> | ||
| Line 12: | Line 10: | ||
|- | |- | ||
| style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 10px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |[[Centre for Regulatory Innovation|<span style="color: #252F38">Home</span>]] | | style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 10px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |[[Centre for Regulatory Innovation|<span style="color: #252F38">Home</span>]] | ||
| − | | style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color:#252F38">[[ | + | | style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color:#252F38">[[Regulatory Sandboxes]]</span> |
| style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[What is Regulatory Experimentation?]]</span> | | style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[What is Regulatory Experimentation?]]</span> | ||
| style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[Regulatory Experimentation Expense Fund]]</span> | | style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[Regulatory Experimentation Expense Fund]]</span> | ||
| style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[Regulators' Capacity Fund]]</span> | | style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[Regulators' Capacity Fund]]</span> | ||
| − | ! style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[ | + | ! style="border-right: white 1px solid; padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[CRI Supported Projects]]</span> |
| style="padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[CRI Tools and Resources |Tools and Resources]]</span> | | style="padding-right: 0px; padding-left: 0px; padding-bottom: 16px; padding-top: 16px; text-align: center; font-family: 'Helvetica Neue', Helvetica, Arial, sans-serif !important;font-size: 11pt;line-height:1.1;font-weight:bold;" width="11%" |<span style="color: #252F38">[[CRI Tools and Resources |Tools and Resources]]</span> | ||
|} | |} | ||
| − | |||
| − | |||
| − | =='''Project Eligibility'''== | + | ==='''Project Eligibility'''=== |
The Regulatory Experimentation Expense Fund (REEF) is a fund to help regulators innovate or support innovation in the marketplace by experimenting with: | The Regulatory Experimentation Expense Fund (REEF) is a fund to help regulators innovate or support innovation in the marketplace by experimenting with: | ||
| Line 30: | Line 26: | ||
<p> | <p> | ||
| − | *determining whether an experiment would be the right approach | + | * determining whether an experiment would be the right approach |
| − | *developing a regulatory experiment (e.g., scope, evidence needs, experimental process, data collection methods | + | *developing a regulatory experiment (e.g., scope, evidence needs, experimental process, data collection methods) |
*conducting a regulatory experiment</p> | *conducting a regulatory experiment</p> | ||
| − | =='''What can the REEF offer?'''== | + | ==='''What can the REEF offer?'''=== |
Regulators can receive: | Regulators can receive: | ||
| Line 41: | Line 37: | ||
*technical advice and guidance to help you navigate your specific situation. | *technical advice and guidance to help you navigate your specific situation. | ||
| + | <!-- Content to be edited for next REEF cycle | ||
='''Interested in applying? Want to learn more?'''= | ='''Interested in applying? Want to learn more?'''= | ||
| − | The first step is to contact the [mailto:cri-cir@tbs-sct.gc.ca?Subject=Regulatory%20Experimentation%20Expense%20Fund%20Enquiry&body=%20 | + | The first step is to contact the [mailto:cri-cir@tbs-sct.gc.ca?Subject=Regulatory%20Experimentation%20Expense%20Fund%20Enquiry&body=%20] to request an informal consultation to determine whether and how the REEF can best support your goals. |
Several different types of projects have been eligible for the REEF – read more [[CRI Supported Projects|here]]. | Several different types of projects have been eligible for the REEF – read more [[CRI Supported Projects|here]]. | ||
| + | --> | ||
| − | + | ==='''The REEF Process'''=== | |
| − | ='''The REEF Process'''= | ||
[[File:The_REEF_Process_Graphic_v2.png|alt=|799x799px]] | [[File:The_REEF_Process_Graphic_v2.png|alt=|799x799px]] | ||
{| class="mw-collapsible mw-collapsed" data-expandtext="Show Detailed Steps" data-collapsetext="Hide Detailed Steps" | {| class="mw-collapsible mw-collapsed" data-expandtext="Show Detailed Steps" data-collapsetext="Hide Detailed Steps" | ||
| Line 54: | Line 51: | ||
|- | |- | ||
| | | | ||
| − | =='''Step 2: Expression of Interest'''== | + | ===='''Step 2: Expression of Interest'''==== |
| − | + | Departments submit a high-level [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2F1%2F17%2FExpression_of_Interest_%2528EOI%2529_Template_.docx&wdOrigin=BROWSELINK Expression of Interest (EOI)] that is used by the CRI to determine the eligibility of the project. To be eligible for funding, proposed experiments must clearly demonstrate how their funding request meets the criteria as outlined in the [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fd%2Fdd%2FREEF_Guide_-_2022.docx&wdOrigin=BROWSELINK REEF Guide]. It is highly recommended that applicants informally confirm the eligibility of their project by [mailto:cri-cir@tbs-sct.gc.ca?Subject=Regulatory%20Experimentation%20Expense%20Fund%20Consultation&body=%20 <u>scheduling</u>] an informal consultation with the CRI before completing an EOI. EOI submissions are due '''TBD.'''<p class="highlighted mw-collapsible-content"></p> | |
| − | |||
| − | |||
| − | Departments submit a high-level [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2F1%2F17%2FExpression_of_Interest_%2528EOI%2529_Template_.docx&wdOrigin=BROWSELINK Expression of Interest (EOI)] that is used by the CRI to determine the eligibility of the project. To be eligible for funding, proposed experiments must clearly demonstrate how their funding request meets the criteria as outlined in the [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fd%2Fdd%2FREEF_Guide_-_2022.docx&wdOrigin=BROWSELINK REEF Guide]. It is highly recommended that applicants informally confirm the eligibility of their project by scheduling an informal consultation with the CRI before completing an EOI. EOI submissions are due ''' | ||
<p class="highlighted inline mw-collapsible-content mw-collapsed">Any Government of Canada regulator may apply for funding. Priority will be given to experiments where regulators have identified collaboration with regulated entities or other businesses with the aim of bringing applications of new and emerging technologies into the Canadian marketplace or enhancing competitiveness.</p> | <p class="highlighted inline mw-collapsible-content mw-collapsed">Any Government of Canada regulator may apply for funding. Priority will be given to experiments where regulators have identified collaboration with regulated entities or other businesses with the aim of bringing applications of new and emerging technologies into the Canadian marketplace or enhancing competitiveness.</p> | ||
| Line 64: | Line 58: | ||
<p class="highlighted inline mw-collapsible-content mw-collapsed"></p> | <p class="highlighted inline mw-collapsible-content mw-collapsed"></p> | ||
| − | <p class="highlighted inline mw-collapsible-content mw-collapsed">The EOI provides a brief overview of the proposed experiment or pre-experimentation project and amount of funding requested. The EOI should not take more than a few hours to complete. If you have questions on how to complete the EOI, it is recommended that you reach out to the CRI. EOIs should be submitted by email to cri-cir@tbs-sct.gc.ca. The CRI will contact applicants as required if clarifications on the proposed experiment or pre-experimentation project are needed. </p> | + | <p class="highlighted inline mw-collapsible-content mw-collapsed">The EOI provides a brief overview of the proposed experiment or pre-experimentation project and amount of funding requested. The EOI should not take more than a few hours to complete. If you have questions on how to complete the EOI, it is recommended that you reach out to the CRI. EOIs should be submitted by email to [mailto:cri-cir@tbs-sct.gc.ca <u>cri-cir@tbs-sct.gc.ca </u>]. The CRI will contact applicants as required if clarifications on the proposed experiment or pre-experimentation project are needed. </p> |
|- | |- | ||
| − | | | + | | |
| − | =='''Step 3: Proposal Submission'''== | + | ===='''Step 3: Proposal Submission'''==== |
|- | |- | ||
|Successful EOI applicants will be invited to complete a full [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fa%2Fa6%2FProposal_Template.docx&wdOrigin=BROWSELINK proposal]. Before developing a proposal, applicants should obtain the support of their Director General or above. The CRI supports applicants throughout the proposal development process. Regulators should refer to the [[:en:images/6/6b/CRI_Regulators'_Experimentation_Toolkit.pdf|Regulators’ Experimentation Toolkit]] to assist in identifying, designing, and carrying out regulatory experiments and sandboxes.<p class="highlighted inline mw-collapsible-content"></p> | |Successful EOI applicants will be invited to complete a full [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fa%2Fa6%2FProposal_Template.docx&wdOrigin=BROWSELINK proposal]. Before developing a proposal, applicants should obtain the support of their Director General or above. The CRI supports applicants throughout the proposal development process. Regulators should refer to the [[:en:images/6/6b/CRI_Regulators'_Experimentation_Toolkit.pdf|Regulators’ Experimentation Toolkit]] to assist in identifying, designing, and carrying out regulatory experiments and sandboxes.<p class="highlighted inline mw-collapsible-content"></p> | ||
| Line 76: | Line 70: | ||
|- | |- | ||
| | | | ||
| − | =='''Step 4: Funding Decision'''== | + | ===='''Step 4: Funding Decision'''==== |
|- | |- | ||
| − | | Funding will be available from April | + | |Funding will be available from April 2025 upon completion of a MOU with the successful applicant and TBS, and is made available on a cost recovery basis.<p class="highlighted inline mw-collapsible-content"></p> |
<p class="highlighted inline mw-collapsible-content mw-collapsed">The department is responsible to forecast their expenses and include the spending schedule in the MOU. The department will be expected to submit scheduled invoices to TBS to recover the expenses incurred as well as submitting financial forecast to allow TBS to decommit the funds that the department no longer plans on recovering.</p><p class="highlighted inline mw-collapsible-content mw-collapsed"></p><p class="highlighted inline mw-collapsible-content mw-collapsed">For more information on each step of the process, please see the [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fd%2Fdd%2FREEF_Guide_-_2022.docx&wdOrigin=BROWSELINK REEF Guide.]</p> | <p class="highlighted inline mw-collapsible-content mw-collapsed">The department is responsible to forecast their expenses and include the spending schedule in the MOU. The department will be expected to submit scheduled invoices to TBS to recover the expenses incurred as well as submitting financial forecast to allow TBS to decommit the funds that the department no longer plans on recovering.</p><p class="highlighted inline mw-collapsible-content mw-collapsed"></p><p class="highlighted inline mw-collapsible-content mw-collapsed">For more information on each step of the process, please see the [https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fd%2Fdd%2FREEF_Guide_-_2022.docx&wdOrigin=BROWSELINK REEF Guide.]</p> | ||
|} | |} | ||
| − | ==Application Documents== | + | ===Application Documents=== |
*[https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fd%2Fdd%2FREEF_Guide_-_2022.docx&wdOrigin=BROWSELINK REEF Guide] | *[https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fd%2Fdd%2FREEF_Guide_-_2022.docx&wdOrigin=BROWSELINK REEF Guide] | ||
*[[:en:images/6/6b/CRI_Regulators'_Experimentation_Toolkit.pdf|Regulators’ Experimentation Toolkit]] | *[[:en:images/6/6b/CRI_Regulators'_Experimentation_Toolkit.pdf|Regulators’ Experimentation Toolkit]] | ||
| − | *[https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages% | + | *[https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwiki.gccollab.ca%2Fimages%2Fc%2Fc2%2FExpression_of_Interest_%2528EOI%2529_Template.docx&wdOrigin=BROWSELINK Expression of Interest (EOI) Template] |
| − | * | + | *[[Media:REEF Proposal Template.docx|Proposal Template]] |
| − | < | + | <h3 id="ongoingprojects">REEF Ongoing Projects</h3> |
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
| Line 104: | Line 98: | ||
|Light Sport Aircraft | |Light Sport Aircraft | ||
|This experiment as proposed by TC involves setting up a sandbox to determine if Light Sport Aircraft, with appropriate conditions, are as reliable as other aircraft currently being used for the purpose of flight training and if the quality of training is as good, or better, than other aircraft currently used for pilot training. The experiment will also inform Transport Canada on potential additional conditions/measures that should be included in future exemptions or regulatory amendments. | |This experiment as proposed by TC involves setting up a sandbox to determine if Light Sport Aircraft, with appropriate conditions, are as reliable as other aircraft currently being used for the purpose of flight training and if the quality of training is as good, or better, than other aircraft currently used for pilot training. The experiment will also inform Transport Canada on potential additional conditions/measures that should be included in future exemptions or regulatory amendments. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- bgcolor="#C8D0C1" | |- bgcolor="#C8D0C1" | ||
|Health Canada (HC) | |Health Canada (HC) | ||
| − | | | + | |Advanced Therapeutic Products Framework for CAR-T Products Manufactured at the Point of Care |
| − | | | + | |The objective of the project is to test the use of a co-creation process to develop tailored requirements for a regulatory sandbox for HC’s Advanced Therapeutic Products pathway, for CAR-T products manufactured at point of care. |
|- | |- | ||
! colspan="3" |[[CRI Supported Projects|Click here to see a list of the CRI’s '''completed''' '''projects''' and experimentation reports]] | ! colspan="3" |[[CRI Supported Projects|Click here to see a list of the CRI’s '''completed''' '''projects''' and experimentation reports]] | ||
Revision as of 14:29, 5 September 2024
| Home | Regulatory Sandboxes | What is Regulatory Experimentation? | Regulatory Experimentation Expense Fund | Regulators' Capacity Fund | CRI Supported Projects | Tools and Resources |
|---|
Project Eligibility
The Regulatory Experimentation Expense Fund (REEF) is a fund to help regulators innovate or support innovation in the marketplace by experimenting with:
- Innovative approaches to any stage(s) of the regulatory lifecycle (issue definition and instrument choice, regulatory development, administration, compliance/enforcement, and review/evaluation)
- Market innovations (e.g., products, business models, services.)
All stages of regulatory experimentation are supported, including:
- determining whether an experiment would be the right approach
- developing a regulatory experiment (e.g., scope, evidence needs, experimental process, data collection methods)
- conducting a regulatory experiment
What can the REEF offer?
Regulators can receive:
- financial support to offset expenses associated with experimenting. Up to $1,400,000 per fiscal year with multi-year funding is available.
- technical advice and guidance to help you navigate your specific situation.
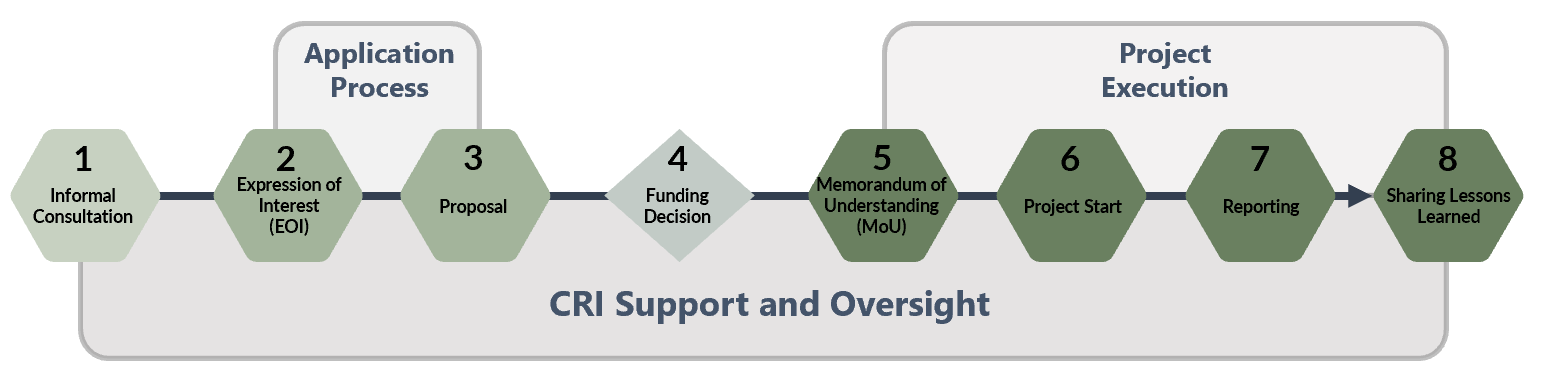
The REEF Process
Step 2: Expression of InterestDepartments submit a high-level Expression of Interest (EOI) that is used by the CRI to determine the eligibility of the project. To be eligible for funding, proposed experiments must clearly demonstrate how their funding request meets the criteria as outlined in the REEF Guide. It is highly recommended that applicants informally confirm the eligibility of their project by scheduling an informal consultation with the CRI before completing an EOI. EOI submissions are due TBD.Any Government of Canada regulator may apply for funding. Priority will be given to experiments where regulators have identified collaboration with regulated entities or other businesses with the aim of bringing applications of new and emerging technologies into the Canadian marketplace or enhancing competitiveness. The EOI provides a brief overview of the proposed experiment or pre-experimentation project and amount of funding requested. The EOI should not take more than a few hours to complete. If you have questions on how to complete the EOI, it is recommended that you reach out to the CRI. EOIs should be submitted by email to cri-cir@tbs-sct.gc.ca . The CRI will contact applicants as required if clarifications on the proposed experiment or pre-experimentation project are needed. |
Step 3: Proposal Submission |
| Successful EOI applicants will be invited to complete a full proposal. Before developing a proposal, applicants should obtain the support of their Director General or above. The CRI supports applicants throughout the proposal development process. Regulators should refer to the Regulators’ Experimentation Toolkit to assist in identifying, designing, and carrying out regulatory experiments and sandboxes.
Proposals will be assessed, and final funding decisions will be determined by an interdepartmental Steering Committee, made up of representatives from TBS, Privy Council Office, Innovation Science and Economic Development, Transport Canada, Environment and Climate Change Canada, Health Canada, and the Community of Federal Regulators. |
Step 4: Funding Decision |
| Funding will be available from April 2025 upon completion of a MOU with the successful applicant and TBS, and is made available on a cost recovery basis.
The department is responsible to forecast their expenses and include the spending schedule in the MOU. The department will be expected to submit scheduled invoices to TBS to recover the expenses incurred as well as submitting financial forecast to allow TBS to decommit the funds that the department no longer plans on recovering. For more information on each step of the process, please see the REEF Guide. |
Application Documents
REEF Ongoing Projects
| Department/ Agency | Project Title | Project Description |
| Transport Canada (TC) | Light Sport Aircraft | This experiment as proposed by TC involves setting up a sandbox to determine if Light Sport Aircraft, with appropriate conditions, are as reliable as other aircraft currently being used for the purpose of flight training and if the quality of training is as good, or better, than other aircraft currently used for pilot training. The experiment will also inform Transport Canada on potential additional conditions/measures that should be included in future exemptions or regulatory amendments. |
| Health Canada (HC) | Advanced Therapeutic Products Framework for CAR-T Products Manufactured at the Point of Care | The objective of the project is to test the use of a co-creation process to develop tailored requirements for a regulatory sandbox for HC’s Advanced Therapeutic Products pathway, for CAR-T products manufactured at point of care. |
| Click here to see a list of the CRI’s completed projects and experimentation reports | ||
|---|---|---|