Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
MDS Database
This will be a page about the database side of the MDS system - about documenting the fields, decodes, and how the system is used. For information about the user interface and the history of the system, see Medical Device System.
The whole of the MDS system is vast and complex, and covers multiple areas from the applications for device licences, the tracking of establishments, companies and contact lists, finance, lists of physicians for Special Access Program, Incidents, and so on. It would be inefficient to try to cover all these various uses in a single go, so instead these will be broken out by the overall sections of the database.
Applications
Describe Applications here
Certificates
Describe certificates here
Companies
Describe Company table relationships here
Devices
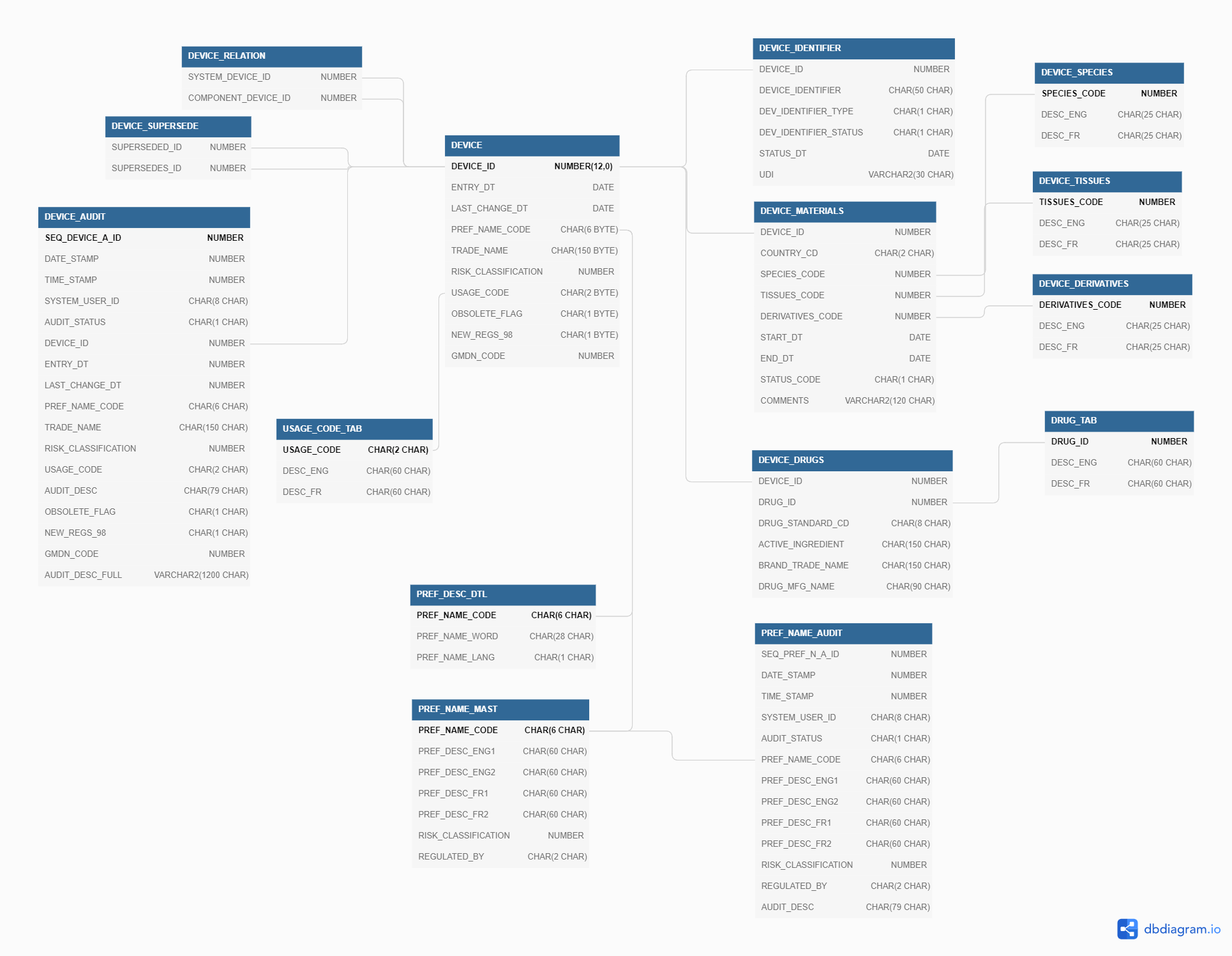
The “Device” tables consist mostly of those tables starting with the term “DEVICE” as well as a few tables that are used to decode these; a few “Dev” tables are actually paired instead with the APPLICATION tables. There will be some overlap as a decode table may be used by more than one set of tables. The following are the tables that are described in this section.
- DEVICE
- DEVICE_AUDIT
- DEVICE_IDENTIFIER
- DEVICE_SUPERSEDE
- DEVICE_RELATION
- DEVICE_MATERIALS
- DEVICE_SPECIES
- DEVICE_TISSUES
- DEVICE_DERIVATIVES
- DEVICE_SAP
- DEVICE_DRUGS
- DRUG_TAB
- USAGE_CODE_TAB
- PREF_NAME_MAST
- PREF_NAME_AUDIT
- PREF_DESC_DTL
Device tables are those for which the DEVICE_ID is the basis for lookup, and that describe the characteristic of devices. Information related to the licence is not directly related to the device, and is captured elsewhere.
DEVICE
The device ID is the basic unit for a medical device, and is used to connect to other tables. Relating an application to devices, for example, makes use of the DEVICE_ID, and this is the table that defines characteristics that are 1-to-1 with the device in this sense. These include the trade name of the device, the risk class under the Medical Devices Regulations, and terms used to group devices together (PNC, GMDN code, and usage code as examples).
DEVICE_AUDIT
The DEVICE_AUDIT table captures the history of changes made to device tables such as adding or removing companies, addition of new device identifiers, etc. As it is an audit table, it has many more rows than the base tables do, and is not efficient to use in most queries of the system.
DEVICE_IDENTIFIER
DEVICE_SUPERSEDE
DEVICE_RELATION
DEVICE_MATERIALS
DEVICE_SPECIES
DEVICE_TISSUES
DEVICE_DERIVATIVES
DEVICE_SAP
DEVICE_DRUGS
DRUG_TAB
USAGE_CODE_TAB
PREF_NAME_MAST
PREF_NAME_AUDIT
PREF_DESC_DTL
Establishments
Establishments go here
Incidents
Incidents go here