Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
Medical Device System
Medical Device System (MDS)
The Medical Device System (often abbreviated to MDS) is the data base (see MDS Database for information specific to the database tables) and graphical user interface used to house medical device program related data such as companies, company contacts, applications for authorization, lists of devices, and more. It is also the source of the data for the Medical Devices Active Licence Listing (MDALL), the outwardly visible database of licensed devices.
The MDS is divided up into sections; this related mostly to the function, but there is substantial communication between the sections (e.g., while the Company section maintains information about companies, other sections like Applications or Incidents will refer to the company entries.)
History
Prior to April 2018, the MDS was run on HP PowerHouse, and this interface and system was rather dated and due to the departure of staff knowledgeable about the language, impossible to update and maintain. The system had been labeled a legacy system that required replacement. In April 2018, the new interface was launched, an HTML5 web-based interface that was more modern and allowed continued development. The system runs on a Oracle database - it was on version 11g for many years and was updated in 2021 to Oracle 19c.
In the old PowerHouse system, each section of the user interface was accessed by numbers, and some fields still have these references in the HTML version or in documentation (e.g., the numbers before the sub-sections on the device screens refer to the field numbers in the old MDS system).
Interface sections
- Device - for maintaining device information
- Company - for maintaining company information
- Meeting
- Application - for maintaining application information
- Special Access - for maintaining information relevant to Special Access Program requests for medical devices
- Correspondence - for maintaining correspondence records
- Quality System
- Batch - used to launch batch processes for administrative closure of incidents
- Incident - for tracking complaints, recalls, and reported medical device problems (Some of these were migrated to the Canada Vigilance ArisG system in February 2020)
- Establishment - for establishment information
- Maintenance
- Logout - log out of the system
Device Section
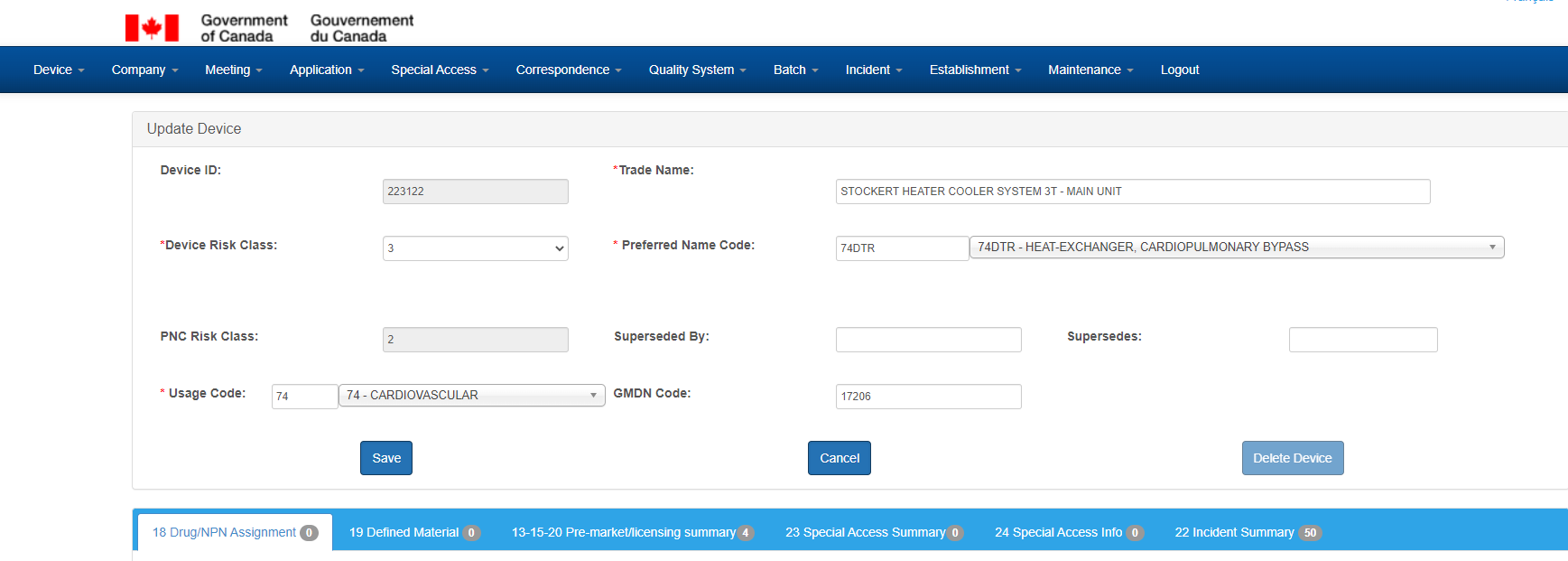
The Device screen is used to maintain device records, and has the following sections - a tombstone section, with general characteristics about the device, accompanied by 6 sub sections:
- Drug/NPN Assignment
- Defined Material
- Pre-market/licensing summary
- Special Access Summary
- Special Access Info
- Incident Summary
Drug/NPN Assignment
This section is used to capture information about drugs that are part of the device; this could include eluted drugs (e.g., paclitaxel eluting stents) as well as drugs applied with the device (disinfecting wipes).
Defined Material
Defined materials are biologically sourced; this section captures information about the source of biological components, including the species (e.g., bovine), tissue (e.g., ligament), and if it is a derivative (e.g., collagen), as well as the country from which it is sourced and can have multiple records for different time periods.
Pre-market/licensing summary
text
Special Access Summary
Text
Special Access Info
Text
Incident Summary
Text