Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
ROEB-Digital Transformation
ROEB Transformation is a movement to evolve the Regulatory Operations and Enforcement Branch (ROEB) at Health Canada into the digital era, by all employees for all Canadians.
Today’s regulatory landscape is complex and continually evolving, through rapid scientific innovation, technological advances and global supply chains. ROEB is committed to modernizing and transforming its compliance and enforcement approach to become more agile, assertive, consistent, innovative, proactive and risk-based. The strategic plan sets the vision for being a world class compliance and enforcement leader, as supported by the Multi-year C&E Transformation plan, which directs and coordinates transformation efforts across ROEB’s compliance and enforcement programs. Successful implementation of the MYCET will require consistent, dedicated leadership and accountability from management, including oversight and reporting at the portfolio level.
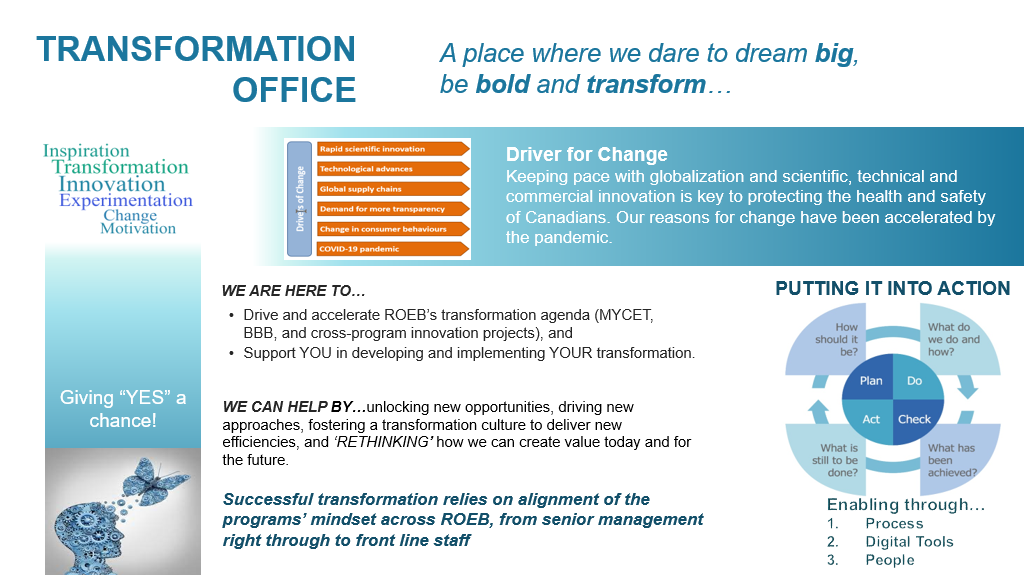
Successful transformation relies on the alignment of compliance and enforcement programs’ mindset across the branch, from senior management right through to the front line staff. To do this, the ROEB Transformation Office was created.
Technology Being Tested
Natural Language Processing (Artificial Intelligence):
Robotics & Remote Sensing (Drones and Satellites)
As part of a Solutions Fund project called Hummingbird, ROEB has been testing data collected using drones and satellites to assess value when applied to outdoor cannabis inspections. To find out more, please visit the Hummingbird repo.
Computer Vision (Artificial Intelligence):
Virtual Reality:
Augmented Reality (mixed reality):
Social Media Monitoring (Artificial Intelligence):
Kelpie
Data Lakes (Data Sharing):
Windows Virtual Desktop Environment:
Geographic Information Systems:
Web Scraping
Technology Being Piloted
Low Code / No Code Automation (Appian)
Robotic Process Automation
- pilot with UiPath (Medical Device)
- pilot with Blueprism (ALR)
- tech assessment/OA and procurement
Digital Notebooks
POD-TBI is currently experimenting with “SceneDoc” application, a Digital Notebook solution created and designed with regulatory officers and their needs in mind. This mobile software fills many of the gaps that currently exist for ROEB C&E inspectors in the field, including their ability to effectively and efficiently collect, track, and store information from an inspection using smartphone or tablet. The SceneDoc – Digital Notebook would also provide a solution for extracting digital content, such as pictures, video and other multimedia from smartphones.
Technology Being Used
- Vidcruiter
Enterprise Investment Plans
IP 628
There are a number of different post-market programs at Health Canada that authorize applicants to conduct regulated activities, against regulated products, at specific locations. These include:
- Medical Device Establishment Licensing (RORB)
- Natural Health Product Site Licensing (HPFB)
- Controlled Substances Dealer Licensing (HECSB)
- Drug Establishment Licensing (RORB)
There are two active IP projects (CSPS and e-CES) that include this functionality. It is also expected that the Cannabis legalization program will require this functionality.
Most programs in this space are using systems with growing aging IT risks, or are still having to use paper and spreadsheet based processes.
Intended direction is to identify and acquire a highly configurable COTS product that is targeted at the regulatory licensing space. This solution will be available to all programs as their primary option for this capability.
Functionality that is within scope of solution is:
- application intake, review, and triage
- inspections that support licence decisions
- risk assessment
- decision workflow
- license/permit generation and notification
- calculation & collection of license fees (cost recovery)
IP 634
IP 701: Border IM/IT Modernization
Invest in a modern IM/IT system for the Health Products Border Program to bring their data collection and management processes onto a single mobile platform, with the following key features:
- Workload management
- Process standardization
- Data quality and structure
- Enterprise collaboration
- Reporting
This IT system will replace two Lotus Notes databases and numerous Excel spreadsheets being used by the health products border program. This initiative is closely linked with Business Analysis/Risk Intelligence initiatives outlined above.
+ other projects like MERLIN & CS & TVCEP
IP 704: Quality Management System
System for Quality Management (ISO 9001:2000): is part of a suite of databases designed specifically to meet the requirements of ISO 9001:2000, the QSI System for Quality Management helps an organization reap the benefits of ISO certification, including improved customer satisfaction and reduced costs. QSi, the software suite used to manage a Quality Management System (QMS) is currently unsupported and is housed on an Aging IT Platform (Lotus Notes). The software suite is inherently unstable and contains many bugs. The RORB programs using QSi (Labs, HPCD, MDCCD) are concerned of a system collapse and how it could potentially impact current Accreditation and Mutual Recognition Agreements in place.
IP 705: DAS Modernization
Modernization of the DAS Labs business proecess and introduction of an end-to-end IT solution for the submission, triage, assessment, and issuance of the DAS Certificate of Analysis.
DHPID Updates