Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
Difference between revisions of "Let’s Innovate Together"
Emma.hughson (talk | contribs) (added workshop and image) |
Emma.hughson (talk | contribs) |
||
| Line 39: | Line 39: | ||
== Workshop Session == | == Workshop Session == | ||
| − | [[File:Workshop.png|thumb|441x441px| | + | [[File:Workshop.png|thumb|441x441px|Workshop Programme |
| + | ]] | ||
The afternoon was dedicated to allow participants gain hands on experience applying a ‘design model’ to problem framing and idea generation. It focused on a process that put the emphasis on understanding the problem through an exploration of the persona – or the person that owns the problem. This is an approach to ensure that the right problem is being solved, and not the symptom(s). | The afternoon was dedicated to allow participants gain hands on experience applying a ‘design model’ to problem framing and idea generation. It focused on a process that put the emphasis on understanding the problem through an exploration of the persona – or the person that owns the problem. This is an approach to ensure that the right problem is being solved, and not the symptom(s). | ||
| Line 56: | Line 57: | ||
== Results from Toronto == | == Results from Toronto == | ||
| + | The Toronto co-design workshop was on February 26, 2020, at 2301 Midland Avenue. Sixteen registered participants attended the session, along with six facilitators and presenters (Martin Bernard, Peter Yoon, Amy McDonald, Sarah Kennedy, Scott McNaughton, Fariba Anderson). The session had 23 people who registered; due to weather and various operational pressures, some were not able to attend. As part of the registration process enabled by [https://www.eventbrite.com/ Eventbrite], participants were asked to submit a problem or opportunity they are passionate about. The following is the list provided by many that registered prior to the workshop. | ||
| + | # The time it takes to perform general drug screening for samples takes too long. | ||
| + | # Foreign paper based inspections could use innovation. | ||
| + | # Case management record keeping is disjointed; needs modernization. | ||
| + | # Buy in for a project that may not be a priority for the stakeholders due to other urgent priorities. | ||
| + | # Maintenance of the facilities that everyone uses in the laboratory. | ||
| + | # Streamlining the initial inspection process (based on risk). Initial inspections are added work on top of regular scheduled inspections. Is it always necessary to go onsite? | ||
| + | # Site inspection note taking is a time-intensive activity. Are there ways to improve the process? | ||
| + | # Working within the resources and parameters of being in the public sector, how do we adapt our training and processes so that we can work with the public while not completely overhauling all the systems and policies that currently exist? | ||
| + | # Reporting tools that we use are very primitive and do not support simultaneous use by multiple users. | ||
| + | # Are there better ways to connect to our stakeholders to execute outreach activities? | ||
| + | # Unable to increase CMP as a trusted or #1 source of public information for risk management. | ||
| + | # Shared Drives: there is a lot of duplicate folders and documents in multiple locations in the shared drives. Is there a better way? | ||
| + | During the afternoon portion of the workshop, three teams of 5 or 6 were formed to apply a hybrid ‘design thinking’ approach. This approach combined Human Centric Design and the Double Diamond Design models. The goal was to help participants feel comfortable with a different process/approach to problem solving. Each group was asked to choose one of the 12 submitted problems/opportunities; they were also given the choice to present a new problem if desired. The following are the summaries from the exercise. | ||
| + | {| class="wikitable" | ||
| + | |+ | ||
| + | !Team Name | ||
| + | !Problem | ||
| + | !Persona | ||
| + | !Impact | ||
| + | !Pitch | ||
| + | |- | ||
| + | |KZPMK (KZ) | ||
| + | |Inability to compile and share information in real-time. The Environmental Health Program compiles activities by each region. Each region has its own ‘system’ to track this data; consolidation for reporting is incredibly time consuming and has potentials for human error as the synthesis is done manually. How can we support a decentralized model to collect and track data with improved accuracy and speed? | ||
| + | |Environmental Health Officer | ||
| + | |Ability to generate accurate reports, in real-time and improve efficiency and productivity. | ||
| + | |Auto file consolidation “dropbox” (auto reconciliation). A data synthesis system that allows regions to drop/submit files in any data format; generates standardized reports required for various stakeholders without the need for any human intervention. | ||
| + | |- | ||
| + | |Storm | ||
| + | |How might reducing the time to take inspection notes for inspectors in order to increase productivity and efficiency for inspectors? (Site inspection note taking is a time-intensive activity. Are there ways to improve the process?) | ||
| + | |public, inspector, management, industry, HQ | ||
| + | |Shorter inspection times; more “inspections” complete; quality of reports; collaboration with industry | ||
| + | |A program that reads all company documents and generates a report. | ||
| + | |- | ||
| + | |Vanilla# | ||
| + | |(Start) Case management record keeping is disjointed; needs modernization. | ||
| + | |ROEB Employees Steve and Alison | ||
| + | |accuracy of information available in systems; efficient processes that drive business outcomes; accessibility of real-time information | ||
| + | |An incentivised system to promote individual competition within public service by using training, vacation, and other ‘currencies’ to enable higher outputs. | ||
| + | |} | ||
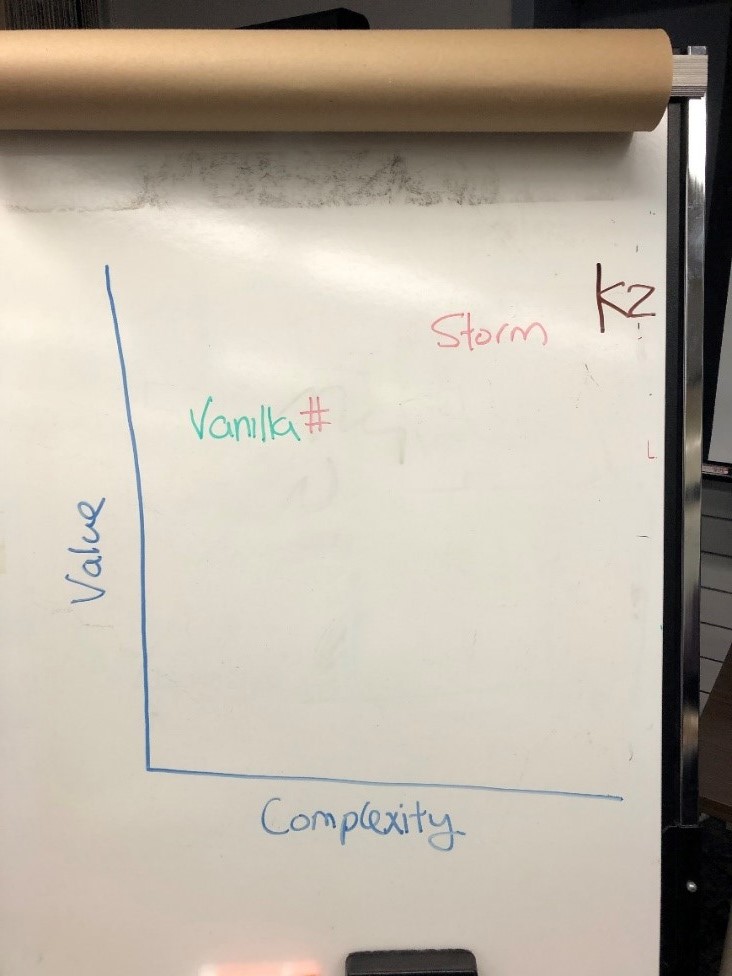
| + | [[File:Wikipic.jpg|thumb|244x244px|Ideas from Toronto session mapped against Value and Complexity.]] | ||
| + | Once the groups presented their problem and idea, it was mapped against two variables: value and complexity. This provided an opportunity to evaluate potential effort and the return on investment. By no means was this exercise scientific. As part of the continuous learning incorporated within design thinking, check points to question and criticize are critical to ensure probability of success. This exercise was used as one mechanism to quickly discuss and evaluate the solution. | ||
== Results from Montreal == | == Results from Montreal == | ||
| + | The Montreal co-design workshop was on February 27, 2020, at 200 Boulevard René Lévesque Ouest, Complexe Guy Favreau. Twelve participants attended the session, along with five facilitators and presenters (Martin Bernard, Peter Yoon, Amy McDonald, Sarah Kennedy, Scott McNaughton). The session had 18 people who registered; due to weather and various operational pressures, some were not able to attend. As part of the registration process enabled by Eventbrite, participants were asked to submit a problem or opportunity they are passionate about. The following is the list provided by many that registered prior to the workshop. | ||
| + | # To list all information of products inspected by hand writing. | ||
| + | # Comment mieux gérer les nombreux et très complexes documents soumis dans le cadre du processus d'évaluation des impacts d'un projet? L'objectif étant d'assurer la transparence et une accessibilité de l'information pour le public. | ||
| + | # Complex laboratory analysis of new substances available on the market. | ||
| + | # As a team lead, a project that we are putting in place to ensure uniformity across country need anti-resistance strategy. Also, the direction seems to be transfer from RORB to OCS during the deployment. | ||
| + | # Environnement de travail sans papier et automatisation de rapport de base de donnée. | ||
| + | # How to better use and/or link databases across the branch in order to complement each other and have more complete and reliable data. | ||
| + | # How to have data available in a more timely manner using automation in order to take action to improve the health of Canadians. | ||
| + | # I’m a new employee at ROEB and I don’t know if I have all the relevant data available to make a reliable decision when regulating health products. It seems that many systems and sources each provide one piece of the puzzle; what I’m missing the big picture. How can I be more confident that I’m using all the right data – and that I can trust that data? | ||
| + | # I found some data that could be valuable for my work but leveraging it isn’t easy – it’s in a proprietary format and I need to do a manual extract and go through several cleansing steps to be able to use it. | ||
| + | # How can I make this more straightforward and easier? | ||
| + | # Équipe dispersée et plusieurs intervenants dans notre programme. | ||
| + | # Le manque de ressources dans un contexte où il y a de multiples priorités à gérer. | ||
| + | # There is no consistency on how tobacco industry keeps track of their internal documentation (No GMP required). Difficult to audit. | ||
| + | # Streamlining the initial inspection process (based on risk). Initial inspections are added work on top of regular scheduled inspections. Is it always necessary to go onsite? | ||
| + | # Is there a better way we can tackle learning about inspections for those that are new? How can we transfer experience from experts to novice employees in a more efficient way? | ||
== Observation & Comments == | == Observation & Comments == | ||
Revision as of 11:13, 23 March 2020
Introduction
Technology and Business Innovation (TBI) under the Planning and Operations Directorate (POD) is responsible for leading the development of the Regulatory Operations and Enforcement Branch (ROEB) investment plan, improving business, and solving problems. Investing in innovation is a major pillar for TBI; it aims to position the Branch as a leader in compliance and enforcement excellence by using innovative approaches to improve regulatory operations.
There is a new Innovation group in TBI called “remic” with a focus on novel approaches, testing via experiments to assess new technologies to potentially modernize and transform ROEB inspection service delivery systems. Remic brings together regulators, researchers, social scientists, data scientists, business and industry experts to collaborate, share, and co-create solutions to support ROEB objectives.
To support capacity building and co-learning within ROEB, TBI planned and executed two workshop sessions with an emphasis on sharing and exploring. The goal was to:
- Promote mindset, skills and behaviours necessary for innovation and experimentation
- Highlight employee-led experimentation (innovation) initiative
- Build knowledge and capacity in ROEB and be leaders in the C&E Transformation
- Nurture ideas into future projects (Submit ideas to Solutions Fund)
During February 2020, TBI visited Toronto and Montreal to facilitate the ‘innovation workshop’ sessions. This report will provide the detailed summary of the workshops and the collected results.
Purpose
In June 2019, the Government of Canada launched a public consultation on a number of initiatives that focused on modernizing the Canadian regulatory system. The intent of the process was to address the regulatory issues by encouraging innovation and competitiveness. A 2016 directive to the Deputy Head of the Federal Departments also ensures that the Government of Canada is committed to devote a fixed percentage of program funds to experimentation that brings new approaches and creates an environment of measurement, evaluation and innovation in program and policy design and delivery. There is a shift in the approach government is taking when investing for the future.
In order to leverage these Government of Canada opportunities ROEB has laid out its 2019-2022 strategic plan that focuses on three key priorities including modernizing the program delivery system, transformation of compliance and enforcement, and achieving excellence in people and workplace. As a result, ROEB is investing in experimentation to advance business processes in the digital era.
Under this plan, ROEB is creating an environment that is conducive to culture shifts to support change and evolution. As well, connecting and sharing with our national Branch partners with a purpose of co-learning and co-creating through experimentation is one of the first steps. The original intent was to visit six cities (Burnaby, Edmonton, Winnipeg, Toronto, Montreal, and Halifax); only Toronto and Montreal was visited – with a plan to visit the other cities in the future.
The purpose of the workshops were the following:
- Share ROEB learnings on experimentation to date, along with information from our corporate partners regarding innovation and experimentation relating to regulatory excellence;
- Expose participants to experimentation methodology, problem framing and design approaches to problem solving; and
- Leverage the workshop as a “launch pad” to co-create with our national partners to solve wicked problems through continuous learning, support, and partnerships.
The workshop was broken up into two portions. In the morning, participants heard presentations from various enablers of experimentation who shared their first-hand experience in developing an innovation project, including the lessons learned and the resources and support available. The workshop presented an introduction to understanding the innovation mindset, framing problems, and the process of experimentation. In the afternoon, participants took part in a hands-on workshop where they collectively chose a problem and applied design thinking principles. The problems they chose were from a list presented by the participants themselves.
The morning programming included the following:
- Share insight from internal and external partners:
- Martin Bernard, Opening Remarks
- Scott McNaughton, Canada School of Public Service, Showcasing Regulatory Innovation
- Fariba Anderson, Gartner, The Language of Change
- Paul Twig, Sierra Systems, Innovation Perspectives from Industry
- Sarah Kennedy, Health Canada’s Solutions Fund: Enabling Experimentation @ HC
- Kimberley Shirley, IMSD: Supporting Experimentation @ HC
- Provide overview on methods and approaches to problem framing and experimentation design
- Peter Yoon, Innovation Mindset, An Open Mindset to Experiment
The morning was to set the stage to provide perspectives from the Government of Canada model to experimentation to the approach adopted by Health Canada. All presentations are available at the TBI GCconnex page: https://gcconnex.gc.ca/file/group/40861893/all#61558995.
Workshop Session
The afternoon was dedicated to allow participants gain hands on experience applying a ‘design model’ to problem framing and idea generation. It focused on a process that put the emphasis on understanding the problem through an exploration of the persona – or the person that owns the problem. This is an approach to ensure that the right problem is being solved, and not the symptom(s).
As part of the process to register, each participant was asked to submit a problem or opportunity they are passionate about. This was used as the case studies for the workshop. The goal was to choose a problem and work through the problem by using the following 10 steps:
- Choosing a Problem
- Identify the Persona
- Refine the Problem
- What are the Needs?
- What can we Leverage?
- Measuring Success
- Brainstorm for Ideas
- Select Ideas for Testing
- The Pitch
- Ranking & Prioritizing
The traditional approach to problem solving tackles problems directly with an attempt to find a solution as quickly as possible. The exercise shifts the traditional approach by leaving the ‘problem solving’ portion near the end of the exercise; instead, the focus is put on understanding the people, their needs, available resources, and how success can be measured. In the following two sections, the results from each of the workshops has been summarized.
Results from Toronto
The Toronto co-design workshop was on February 26, 2020, at 2301 Midland Avenue. Sixteen registered participants attended the session, along with six facilitators and presenters (Martin Bernard, Peter Yoon, Amy McDonald, Sarah Kennedy, Scott McNaughton, Fariba Anderson). The session had 23 people who registered; due to weather and various operational pressures, some were not able to attend. As part of the registration process enabled by Eventbrite, participants were asked to submit a problem or opportunity they are passionate about. The following is the list provided by many that registered prior to the workshop.
- The time it takes to perform general drug screening for samples takes too long.
- Foreign paper based inspections could use innovation.
- Case management record keeping is disjointed; needs modernization.
- Buy in for a project that may not be a priority for the stakeholders due to other urgent priorities.
- Maintenance of the facilities that everyone uses in the laboratory.
- Streamlining the initial inspection process (based on risk). Initial inspections are added work on top of regular scheduled inspections. Is it always necessary to go onsite?
- Site inspection note taking is a time-intensive activity. Are there ways to improve the process?
- Working within the resources and parameters of being in the public sector, how do we adapt our training and processes so that we can work with the public while not completely overhauling all the systems and policies that currently exist?
- Reporting tools that we use are very primitive and do not support simultaneous use by multiple users.
- Are there better ways to connect to our stakeholders to execute outreach activities?
- Unable to increase CMP as a trusted or #1 source of public information for risk management.
- Shared Drives: there is a lot of duplicate folders and documents in multiple locations in the shared drives. Is there a better way?
During the afternoon portion of the workshop, three teams of 5 or 6 were formed to apply a hybrid ‘design thinking’ approach. This approach combined Human Centric Design and the Double Diamond Design models. The goal was to help participants feel comfortable with a different process/approach to problem solving. Each group was asked to choose one of the 12 submitted problems/opportunities; they were also given the choice to present a new problem if desired. The following are the summaries from the exercise.
| Team Name | Problem | Persona | Impact | Pitch |
|---|---|---|---|---|
| KZPMK (KZ) | Inability to compile and share information in real-time. The Environmental Health Program compiles activities by each region. Each region has its own ‘system’ to track this data; consolidation for reporting is incredibly time consuming and has potentials for human error as the synthesis is done manually. How can we support a decentralized model to collect and track data with improved accuracy and speed? | Environmental Health Officer | Ability to generate accurate reports, in real-time and improve efficiency and productivity. | Auto file consolidation “dropbox” (auto reconciliation). A data synthesis system that allows regions to drop/submit files in any data format; generates standardized reports required for various stakeholders without the need for any human intervention. |
| Storm | How might reducing the time to take inspection notes for inspectors in order to increase productivity and efficiency for inspectors? (Site inspection note taking is a time-intensive activity. Are there ways to improve the process?) | public, inspector, management, industry, HQ | Shorter inspection times; more “inspections” complete; quality of reports; collaboration with industry | A program that reads all company documents and generates a report. |
| Vanilla# | (Start) Case management record keeping is disjointed; needs modernization. | ROEB Employees Steve and Alison | accuracy of information available in systems; efficient processes that drive business outcomes; accessibility of real-time information | An incentivised system to promote individual competition within public service by using training, vacation, and other ‘currencies’ to enable higher outputs. |
Once the groups presented their problem and idea, it was mapped against two variables: value and complexity. This provided an opportunity to evaluate potential effort and the return on investment. By no means was this exercise scientific. As part of the continuous learning incorporated within design thinking, check points to question and criticize are critical to ensure probability of success. This exercise was used as one mechanism to quickly discuss and evaluate the solution.
Results from Montreal
The Montreal co-design workshop was on February 27, 2020, at 200 Boulevard René Lévesque Ouest, Complexe Guy Favreau. Twelve participants attended the session, along with five facilitators and presenters (Martin Bernard, Peter Yoon, Amy McDonald, Sarah Kennedy, Scott McNaughton). The session had 18 people who registered; due to weather and various operational pressures, some were not able to attend. As part of the registration process enabled by Eventbrite, participants were asked to submit a problem or opportunity they are passionate about. The following is the list provided by many that registered prior to the workshop.
- To list all information of products inspected by hand writing.
- Comment mieux gérer les nombreux et très complexes documents soumis dans le cadre du processus d'évaluation des impacts d'un projet? L'objectif étant d'assurer la transparence et une accessibilité de l'information pour le public.
- Complex laboratory analysis of new substances available on the market.
- As a team lead, a project that we are putting in place to ensure uniformity across country need anti-resistance strategy. Also, the direction seems to be transfer from RORB to OCS during the deployment.
- Environnement de travail sans papier et automatisation de rapport de base de donnée.
- How to better use and/or link databases across the branch in order to complement each other and have more complete and reliable data.
- How to have data available in a more timely manner using automation in order to take action to improve the health of Canadians.
- I’m a new employee at ROEB and I don’t know if I have all the relevant data available to make a reliable decision when regulating health products. It seems that many systems and sources each provide one piece of the puzzle; what I’m missing the big picture. How can I be more confident that I’m using all the right data – and that I can trust that data?
- I found some data that could be valuable for my work but leveraging it isn’t easy – it’s in a proprietary format and I need to do a manual extract and go through several cleansing steps to be able to use it.
- How can I make this more straightforward and easier?
- Équipe dispersée et plusieurs intervenants dans notre programme.
- Le manque de ressources dans un contexte où il y a de multiples priorités à gérer.
- There is no consistency on how tobacco industry keeps track of their internal documentation (No GMP required). Difficult to audit.
- Streamlining the initial inspection process (based on risk). Initial inspections are added work on top of regular scheduled inspections. Is it always necessary to go onsite?
- Is there a better way we can tackle learning about inspections for those that are new? How can we transfer experience from experts to novice employees in a more efficient way?