Important: The GCConnex decommission will not affect GCCollab or GCWiki. Thank you and happy collaborating!
Difference between revisions of "MDS DEVICE table"
(Created page with "The DEVICE table is the base table for capturing characteristics of medical devices; it has the following 10 columns: {| class="wikitable" |+Fields on the MDSDB.DEVICE table !...") |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | The DEVICE table is the base table for capturing characteristics of medical devices; it has the following 10 columns: | + | The DEVICE table is the base table in the [[MDS Database]] for capturing characteristics of medical devices; it also houses the tombstone data for the [[Medical Device System#Device Section|MDS Device section]] in the user interface has the following 10 columns: |
{| class="wikitable" | {| class="wikitable" | ||
|+Fields on the MDSDB.DEVICE table | |+Fields on the MDSDB.DEVICE table | ||
| Line 68: | Line 68: | ||
|no decode available in system | |no decode available in system | ||
|} | |} | ||
| − | Of these, the DEVICE_ID is the most important, being a consistent and unique identifier used across the system to refer to a device. We also often use the TRADE_NAME, as it is more understandable to humans than DEVICE_IDs are. USAGE_CODE, PREF_NAME_CODE (PNC), and GMDN_CODE are various ways to group devices together, in increasingly precise ways, and RISK_CLASSIFICATION is used as a loose “risk level” of devices. The remaining fields are a pair of dates | + | Of these, the DEVICE_ID is the most important, being a consistent and unique identifier used across the system to refer to a device. We also often use the TRADE_NAME, as it is more understandable to humans than DEVICE_IDs are. USAGE_CODE, PREF_NAME_CODE (PNC), and GMDN_CODE are various ways to group devices together, in increasingly precise ways, and RISK_CLASSIFICATION is used as a loose “risk level” of devices. The remaining fields are a pair of dates, a flag for whether the device is under the “new” regulations, and an “obsolete” flag. |
| − | == DEVICE_ID == | + | === DEVICE_ID === |
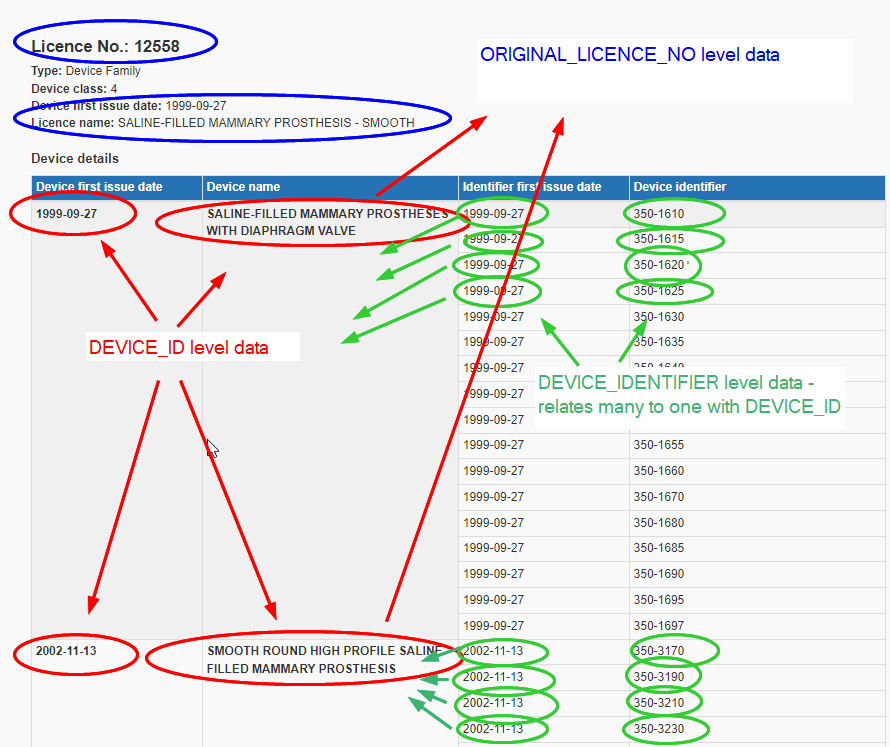
| − | DEVICE_ID is the primary key for this table. The Device ID is a grouping up of sometimes multiple model numbers into a group that one can think of as being the “device”; examples would be a type of breast implant, or a style and type of tubing made by a specific company. Here is a screenshot from the current (2022) MDALL, showing the various levels of the device hierarchy. | + | DEVICE_ID is the primary key for this table. The Device ID is a grouping up of sometimes multiple model numbers into a group that one can think of as being the “device”; examples would be a type of breast implant, or a style and type of tubing made by a specific company. Here is a screenshot from the current (2022) [[Medical Devices Active Licence Listing]] ([https://health-products.canada.ca/mdall-limh/ MDALL]), showing the various levels of the device hierarchy. |
| + | [[File:Device hierarchy.png|thumb|Screenshot from the MDALL illustrating the many-to one relationships between device identifiers, device IDs, and licences.]] | ||
As you can see in this example, the licence is for SALINE-FILLED MAMMARY PROTHESIS – SMOOTH, but it has at least two DEVICE_IDs included, that were issued at different times. Each of these DEVICE_IDs then has many models numbers, chiefly differing in this case in the size of the implant. For tubing or screws, as another example, device identifiers may be the length of the tubing or screws. | As you can see in this example, the licence is for SALINE-FILLED MAMMARY PROTHESIS – SMOOTH, but it has at least two DEVICE_IDs included, that were issued at different times. Each of these DEVICE_IDs then has many models numbers, chiefly differing in this case in the size of the implant. For tubing or screws, as another example, device identifiers may be the length of the tubing or screws. | ||
| Line 77: | Line 78: | ||
At the time of writing, there were close to 300,000 device IDs in the MDS system. This is the main field used to link devices to incidents, to applications, and is often the unit we speak of when we talk about “a device”. | At the time of writing, there were close to 300,000 device IDs in the MDS system. This is the main field used to link devices to incidents, to applications, and is often the unit we speak of when we talk about “a device”. | ||
| − | + | === ENTRY_DT === | |
This is a date field, and captures when the device was added to the DEVICE table. | This is a date field, and captures when the device was added to the DEVICE table. | ||
| Line 84: | Line 85: | ||
ENTRY_DT seems to first appear in 1996, presumably data was migrated from another system to the MDS for these earlier entries. | ENTRY_DT seems to first appear in 1996, presumably data was migrated from another system to the MDS for these earlier entries. | ||
| − | + | === LAST_CHANGE_DT === | |
Another date field, captures modification date of the record. | Another date field, captures modification date of the record. | ||
| Line 91: | Line 92: | ||
This date also first appears in 1996, presumably when the MDS became operational. | This date also first appears in 1996, presumably when the MDS became operational. | ||
| − | + | === PREF_NAME_CODE === | |
| − | This is a way to group devices that have similar function; it is referred to online in the '' | + | This is a way to group devices that have similar function; it is referred to online in the ''[https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/application-information/guidance-documents/guidance-industry-keyword-assist-manufacturers-class-medical-devices.html Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices].'' |
| − | This system of classification essentially uses the FDAs product code groups and the USAGE code to come up with a 5-character code consisting of two digits (the USAGE_CODE) and three letters (resembling the FDA | + | This system of classification essentially uses the [https://www.fda.gov/medical-devices/classify-your-medical-device/product-code-classification-database FDAs product code] groups and the USAGE code to come up with a 5-character code consisting of two digits (the USAGE_CODE) and three letters (resembling the FDA product codes). |
An example PREF_NAME_CODE (PNC) would be 87QJK, which is decoded as CHAIR, PODIATRIC. | An example PREF_NAME_CODE (PNC) would be 87QJK, which is decoded as CHAIR, PODIATRIC. | ||
| Line 100: | Line 101: | ||
The 87 here refers to the USAGE_CODE for orthopaedics, and QJK uniquely identifies podiatric chairs. | The 87 here refers to the USAGE_CODE for orthopaedics, and QJK uniquely identifies podiatric chairs. | ||
| − | The PNC is decoded by the table | + | The PNC is decoded by the table [[MDS PREF NAME MAST table|PREF_NAME_MAST]], but also has keywords on the table [[MDS PREF DESC DTL table|PREF_DESC_DTL]]. There are roughly 5,900 distinct PNCs assigned to devices. |
This field is 100% populated, and appears on every record. | This field is 100% populated, and appears on every record. | ||
| Line 106: | Line 107: | ||
The digits representing the USAGE_CODE on a PNC may not match the USAGE_CODE later on this table; it is not clear whether this is an error, or simply allows for flexibility. I find that the first two digits of the PNC are a better classifier in general, and it avoids selecting outdated USAGE_CODES that have been retired. | The digits representing the USAGE_CODE on a PNC may not match the USAGE_CODE later on this table; it is not clear whether this is an error, or simply allows for flexibility. I find that the first two digits of the PNC are a better classifier in general, and it avoids selecting outdated USAGE_CODES that have been retired. | ||
| − | + | As with many fields in the MDS, it is padded with white space, but as it is only 6 characters long and the PNCs are 5 characters it has little impact on presentation. | |
| − | The TRADE_NAME is the name assigned to the device; it is just a string and is not necessarily unique. | + | |
| + | === TRADE_NAME === | ||
| + | The '''TRADE_NAME''' is the name assigned to the device; it is just a string and is not necessarily unique. As with many fields in the MDS, it is padded with white space. | ||
This field is populated with non-whitespace >99.9% of the time; there are currently (April 2022) 130 device entries with blank TRADE_NAMEs. | This field is populated with non-whitespace >99.9% of the time; there are currently (April 2022) 130 device entries with blank TRADE_NAMEs. | ||
| − | + | === RISK_CLASSIFICATION === | |
| − | The RISK_CLASSIFICATION is the class of the device under the ''Medical Device Regulations''. The only valid options under the regulations are classes I, II, III, or IV; these are entered as the Arabic numerals 1,2,3, and 4 in the MDS system. | + | The '''RISK_CLASSIFICATION''' is the class of the device under the ''[https://laws-lois.justice.gc.ca/eng/regulations/sor-98-282/ Medical Device Regulations]''. The only valid options under the regulations are classes I, II, III, or IV; these are entered as the Arabic numerals 1,2,3, and 4 in the MDS system. |
| − | This field is 100% filled, but there are ~22,000 entries with a RISK_CLASSIFICATION of 0, which is not a valid | + | This field is 100% filled, but there are ~22,000 entries with a RISK_CLASSIFICATION of 0, which is not a valid regulatory class of device. |
| − | The term RISK_CLASSIFICATION appears on other tables, such as the | + | The term RISK_CLASSIFICATION appears on other tables, such as the [[MDS PREF NAME MAST table|PREF_NAME_MAST]] table; in this instance, it refers to the risk classification for the device ID, but on other tables, it may be referring to a group of devices in general or to a licence. |
| − | The risk classification on a device may not match the risk classification of the PNC on the | + | The risk classification on a device may not match the risk classification of the PNC on the PREF_NAME_MAST table; it is not clear whether this is an error, or simply allows for flexibility. |
| − | + | === USAGE_CODE === | |
| − | The USAGE_CODE defines a broad group of associated devices. These categorizations are decoded by the | + | The USAGE_CODE defines a broad group of associated devices. These categorizations are decoded by the [[MDS USAGE CODE TAB table|USAGE_CODE_TAB]] table. |
This field is populated >99.9% of the time with only 82 nulls, however 1% of the entries (3192) are assigned to the USAGE_CODE 00 which is “UNDEFINED” and ~7.5% (21983) belong to retired/old usage codes (these are marked by an asterisk in the decode table). | This field is populated >99.9% of the time with only 82 nulls, however 1% of the entries (3192) are assigned to the USAGE_CODE 00 which is “UNDEFINED” and ~7.5% (21983) belong to retired/old usage codes (these are marked by an asterisk in the decode table). | ||
| − | + | === OBSOLETE_FLAG === | |
I am not certain what this field is for, and have never used it. The field has the values N, Y, and null. Nulls make up ~258,000 entries, accounting for 88% of entries. | I am not certain what this field is for, and have never used it. The field has the values N, Y, and null. Nulls make up ~258,000 entries, accounting for 88% of entries. | ||
| − | + | === NEW_REGS_98 === | |
I am under the impression that this marks whether a device is subject to the new regulations, or was grandfathered in. This field is 100% populated with Y or N. ~39,000 devices are marked “N.” | I am under the impression that this marks whether a device is subject to the new regulations, or was grandfathered in. This field is 100% populated with Y or N. ~39,000 devices are marked “N.” | ||
| − | + | === GMDN_CODE === | |
| − | The GMDN_CODE field was added to the table in 2018, and is the newest field on this table. The intent is to capture a code from the | + | The GMDN_CODE field was added to the table in 2018, and is the newest field on this table. The intent is to capture a code from the [https://www.gmdnagency.org/ Global Medical Device Nomenclature] for devices moving forward, and to add these codes retrospectively to the system. Currently (April 2022) there are ~80,000 devices with non-0 GMDN codes (27%); a further 6,000 or so (2%) have only the number 0 (a default placeholder) and the remaining 208,000 devices (71%) are null. Of those assigned codes, 1,200 are either assigned obsoleted or non-existent codes. |
| − | There are currently ~7,800 distinct GMDN_CODEs entered, out of a list of nearly 30,000 possible GMDN codes. As this is too extensive to provide decodes for in this document, users are invited to download these lists directly from the GMDN site should they need to decode this field. | + | There are currently ~7,800 distinct GMDN_CODEs entered, out of a list of nearly 30,000 possible GMDN codes. As this is too extensive to provide decodes for in this document, users are invited to download these lists directly from the [https://www.gmdnagency.org/Terms/Downloads GMDN site] should they need to decode this field. |
Latest revision as of 15:12, 4 May 2022
The DEVICE table is the base table in the MDS Database for capturing characteristics of medical devices; it also houses the tombstone data for the MDS Device section in the user interface has the following 10 columns:
| Field name | Data Type | Nullable? | Default | Decode |
|---|---|---|---|---|
| DEVICE_ID | NUMBER | No | ||
| ENTRY_DT | DATE | Yes | ||
| LAST_CHANGE_DT | DATE | Yes | ||
| PREF_NAME_CODE | CHAR(6 CHAR) | No | PREF_NAME_MAST | |
| TRADE_NAME | CHAR(150 CHAR) | No | ||
| RISK_CLASSIFICATION | NUMBER | No | ||
| USAGE_CODE | CHAR(2 CHAR) | No | USAGE_CODE_TAB | |
| OBSOLETE_FLAG | CHAR(1 CHAR) | No | ||
| NEW_REGS_98 | CHAR(1 CHAR) | No | 'Y' | |
| GMDN_CODE | NUMBER | Yes | no decode available in system |
Of these, the DEVICE_ID is the most important, being a consistent and unique identifier used across the system to refer to a device. We also often use the TRADE_NAME, as it is more understandable to humans than DEVICE_IDs are. USAGE_CODE, PREF_NAME_CODE (PNC), and GMDN_CODE are various ways to group devices together, in increasingly precise ways, and RISK_CLASSIFICATION is used as a loose “risk level” of devices. The remaining fields are a pair of dates, a flag for whether the device is under the “new” regulations, and an “obsolete” flag.
DEVICE_ID
DEVICE_ID is the primary key for this table. The Device ID is a grouping up of sometimes multiple model numbers into a group that one can think of as being the “device”; examples would be a type of breast implant, or a style and type of tubing made by a specific company. Here is a screenshot from the current (2022) Medical Devices Active Licence Listing (MDALL), showing the various levels of the device hierarchy.
As you can see in this example, the licence is for SALINE-FILLED MAMMARY PROTHESIS – SMOOTH, but it has at least two DEVICE_IDs included, that were issued at different times. Each of these DEVICE_IDs then has many models numbers, chiefly differing in this case in the size of the implant. For tubing or screws, as another example, device identifiers may be the length of the tubing or screws.
At the time of writing, there were close to 300,000 device IDs in the MDS system. This is the main field used to link devices to incidents, to applications, and is often the unit we speak of when we talk about “a device”.
ENTRY_DT
This is a date field, and captures when the device was added to the DEVICE table.
~88% of rows of the table have an entry date (~34,000 rows are null for ENTRY_DT)
ENTRY_DT seems to first appear in 1996, presumably data was migrated from another system to the MDS for these earlier entries.
LAST_CHANGE_DT
Another date field, captures modification date of the record.
~94% of rows of the table have an entry date (~14,000 rows are null for LAST_CHANGE_DT)
This date also first appears in 1996, presumably when the MDS became operational.
PREF_NAME_CODE
This is a way to group devices that have similar function; it is referred to online in the Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices.
This system of classification essentially uses the FDAs product code groups and the USAGE code to come up with a 5-character code consisting of two digits (the USAGE_CODE) and three letters (resembling the FDA product codes).
An example PREF_NAME_CODE (PNC) would be 87QJK, which is decoded as CHAIR, PODIATRIC.
The 87 here refers to the USAGE_CODE for orthopaedics, and QJK uniquely identifies podiatric chairs.
The PNC is decoded by the table PREF_NAME_MAST, but also has keywords on the table PREF_DESC_DTL. There are roughly 5,900 distinct PNCs assigned to devices.
This field is 100% populated, and appears on every record.
The digits representing the USAGE_CODE on a PNC may not match the USAGE_CODE later on this table; it is not clear whether this is an error, or simply allows for flexibility. I find that the first two digits of the PNC are a better classifier in general, and it avoids selecting outdated USAGE_CODES that have been retired.
As with many fields in the MDS, it is padded with white space, but as it is only 6 characters long and the PNCs are 5 characters it has little impact on presentation.
TRADE_NAME
The TRADE_NAME is the name assigned to the device; it is just a string and is not necessarily unique. As with many fields in the MDS, it is padded with white space.
This field is populated with non-whitespace >99.9% of the time; there are currently (April 2022) 130 device entries with blank TRADE_NAMEs.
RISK_CLASSIFICATION
The RISK_CLASSIFICATION is the class of the device under the Medical Device Regulations. The only valid options under the regulations are classes I, II, III, or IV; these are entered as the Arabic numerals 1,2,3, and 4 in the MDS system.
This field is 100% filled, but there are ~22,000 entries with a RISK_CLASSIFICATION of 0, which is not a valid regulatory class of device.
The term RISK_CLASSIFICATION appears on other tables, such as the PREF_NAME_MAST table; in this instance, it refers to the risk classification for the device ID, but on other tables, it may be referring to a group of devices in general or to a licence.
The risk classification on a device may not match the risk classification of the PNC on the PREF_NAME_MAST table; it is not clear whether this is an error, or simply allows for flexibility.
USAGE_CODE
The USAGE_CODE defines a broad group of associated devices. These categorizations are decoded by the USAGE_CODE_TAB table.
This field is populated >99.9% of the time with only 82 nulls, however 1% of the entries (3192) are assigned to the USAGE_CODE 00 which is “UNDEFINED” and ~7.5% (21983) belong to retired/old usage codes (these are marked by an asterisk in the decode table).
OBSOLETE_FLAG
I am not certain what this field is for, and have never used it. The field has the values N, Y, and null. Nulls make up ~258,000 entries, accounting for 88% of entries.
NEW_REGS_98
I am under the impression that this marks whether a device is subject to the new regulations, or was grandfathered in. This field is 100% populated with Y or N. ~39,000 devices are marked “N.”
GMDN_CODE
The GMDN_CODE field was added to the table in 2018, and is the newest field on this table. The intent is to capture a code from the Global Medical Device Nomenclature for devices moving forward, and to add these codes retrospectively to the system. Currently (April 2022) there are ~80,000 devices with non-0 GMDN codes (27%); a further 6,000 or so (2%) have only the number 0 (a default placeholder) and the remaining 208,000 devices (71%) are null. Of those assigned codes, 1,200 are either assigned obsoleted or non-existent codes.
There are currently ~7,800 distinct GMDN_CODEs entered, out of a list of nearly 30,000 possible GMDN codes. As this is too extensive to provide decodes for in this document, users are invited to download these lists directly from the GMDN site should they need to decode this field.